Electrocatalytic N2-to-NH3 conversion with high faradaic efficiency enabled using a Bi nanosheet array†
Abstract
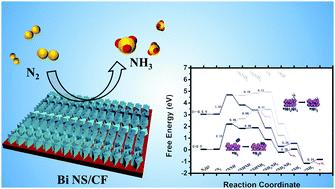
Electrocatalytic N2 reduction represents a promising alternative to the conventional Haber–Bosch process for ambient N2-to-NH3 fixation, but it is severely challenged by competitive hydrogen evolution, which limits the current efficiency for NH3 formation. In this work, a nanosheet array of metallic Bi, an environmentally benign elemental substance previously predicted theoretically to have low hydrogen-evolving activity, is proposed as a superior catalyst for N2 reduction electrocatalysis. Electrochemical tests show that the Bi nanosheet array on Cu foil as a stable 3D catalyst electrode achieves a high faradaic efficiency of 10.26% with an NH3 yield rate of 6.89 × 10−11 mol s−1 cm−2 at −0.50 V vs. the reversible hydrogen electrode in 0.1 M HCl, rivalling the performances of most reported noble-metal-free catalysts operating in acids. Density functional theory calculations suggest that Bi effectively activates the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and the alternating mechanism is energetically favourable.
N bond and the alternating mechanism is energetically favourable.


 Please wait while we load your content...
Please wait while we load your content...