Development of BODIPY dyes with versatile functional groups at 3,5-positions from diacyl peroxides via Cu(ii)-catalyzed radical alkylation†
Abstract
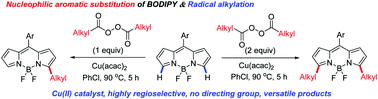
An efficient Cu(II)-catalyzed, C–H alkylation of BODIPY with a variety of alkyl diacyl peroxides has been developed for the first time, providing a late-stage and straightforward method for controllable synthesis of monoalkylated and dialkylated BODIPYs via a radical process that otherwise is difficult to obtain by literature methods. This chemo- and site-selective transformation will allow for the introduction of a variety of functionalities on the BODIPY core for highly versatile tethering to receptors and to other molecules of interest.


 Please wait while we load your content...
Please wait while we load your content...