Access to chiral tetrahydrofluorenes through a palladium-catalyzed enantioselective tandem intramolecular Heck/Tsuji–Trost reaction†
Abstract
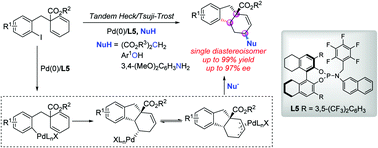
A palladium-catalyzed enantioselective coupling of 2,5-cyclohexadienyl-substituted aryl iodides and carbon or heteroatom nucleophiles is described. The reaction proceeded via a tandem asymmetric Heck insertion and Tsuji–Trost allylation, enabling the rapid construction of valuable chiral tetrahydrofluorenes by using a chiral H8-BINOL-based phosphoramidite ligand.


 Please wait while we load your content...
Please wait while we load your content...