Photooxygenation of 2-propargylfurans: a path to structurally diverse nitrogen-containing 5-membered rings†
Abstract
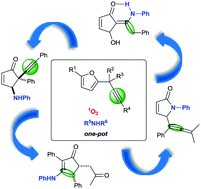
The photooxygenation of 2-propargylfurans enabled access to original nitrogen-containing cyclopentenones and related compounds in a one-pot fashion. By employing readily-available substrates such as furans and amines, we succeeded in achieving a high degree of molecular complexity. Relying on the introduction of an alkyne moiety and tailored substrates, this transformation reveals a new facet for reaction sequences featuring the photooxygenation of furans.


 Please wait while we load your content...
Please wait while we load your content...