Evaluation of excited state bond weakening for ammonia synthesis from a manganese nitride: stepwise proton coupled electron transfer is preferred over hydrogen atom transfer†
Abstract
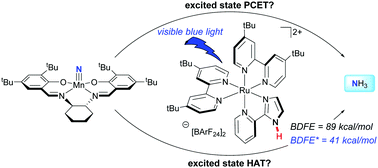
Concepts for the thermodynamically challenging synthesis of weak N–H bonds by photoinduced proton coupled electron transfer are explored. Upon irradiation with blue light, ammonia synthesis was achieved from the manganese nitride (tBuSalen)MnN (tBuSalen = (S,S)-(+)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine) in the presence of 9,10-dihydroacridine and a ruthenium photocatalyst in iPrOH solution. Although in one case the ruthenium complex bears a remote N–H bond that weakens to 41 kcal mol−1 upon irradiation, control experiments with the N-methylated analog demonstrate the ruthenium complex serves as a photoreductant rather than hydrogen-atom transfer catalyst in aprotic solvents. Luminescence quenching experiments support a ruthenium(II)/(III) cycle rather than a ruthenium(I)/(II) alternative. Identification of the manganese complex following ammonia synthesis was also accomplished.
- This article is part of the themed collection: Frontiers in Proton Coupled Electron Transfer (PCET)


 Please wait while we load your content...
Please wait while we load your content...
