Strong positive allosteric cooperativity in ternary complexes based on hydrogen-bonded aromatic amide macrocycles†
Abstract
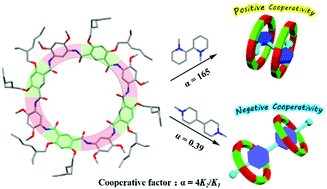
Three new hydrogen-bonded aromatic amide macrocycles with eight residues were synthesized. The first single crystal structure of this class of larger macrocycles was obtained, which reveals a saddle-like conformation. Interestingly, in sharp contrast to previous negative cooperativity in binding paraquat with cyclo[6]aramide, strong positive allosteric cooperativity in ternary complexes was observed. This may open an avenue for the construction of mechanically interlocked molecules with these larger H-bonded macrocycles.


 Please wait while we load your content...
Please wait while we load your content...