A palladium-catalyzed regiocontrollable hydroarylation reaction of allenamides with B2pin2/H2O†
Abstract
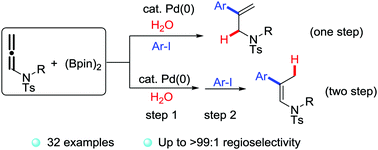
A novel palladium-catalyzed regiocontrollable hydroarylation reaction of allenamides with B2pin2/H2O has been disclosed. H2O as an ideal hydrogen source was activated by B2pin2 to furnish allylamines or enamines with a broad functional group tolerance. The regioselectivity for both of the two products was up to 99 : 1 for most of the examples, which was achieved by adjusting the addition order of the catalyst and iodobenzene derivatives. The tentative investigation of the mechanism proved the reaction to be a non-radical process and the deuterium-labeled experiments indicated that the hydrogen was from H2O.


 Please wait while we load your content...
Please wait while we load your content...