Copper-catalyzed intermolecular oxidative trifluoromethyl-arylation of styrenes with NaSO2CF3 and indoles involving C–H functionalization†
Abstract
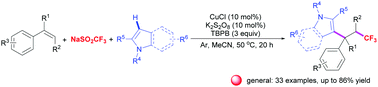
A new copper-catalyzed three-component oxidative 1,2-trifluoromethylarylation of styrenes with NaSO2CF3 and indoles involving aryl C–H bond functionalization is described. This reaction is initiated by single electron transfer upon utilizing TBPB as an oxidant, thus enabling introduction of a CF3 group and an aryl group into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of alkenes in a single step. This method represents a highly atom and step-economical access to various CF3-substituted 1,1-diarylalkane derivatives in moderate to good yields with good functional group compatibility.
C bond of alkenes in a single step. This method represents a highly atom and step-economical access to various CF3-substituted 1,1-diarylalkane derivatives in moderate to good yields with good functional group compatibility.


 Please wait while we load your content...
Please wait while we load your content...