Ruthenium catalyzes the synthesis of γ-butenolides fused with cyclohexanones†
Abstract
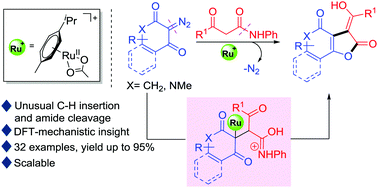
Ruthenium(II)-catalyzed reactions of cyclic diazodicarbonyl compounds with β-ketoamides for chemo- and stereoselective construction of cyclohexanone-fused γ-butenolides are described. This study represents the first example of the addition of an enol substrate which is formed by the tautomerization of the β-ketoamides to the electrophilic carbene center for unusual cyclization through amide cleavage. The combined experimental and computational studies shed light on the mechanistic pathway favouring the unusual ring formation reaction instead of the involvement of the general carbonyl ylide intermediates for the product generation.


 Please wait while we load your content...
Please wait while we load your content...