Cascade Rh(ii) and Yb(iii) catalyzed synthesis of substituted naphthofurans via transannulation of N-sulfonyl-1,2,3-triazoles with β-naphthols†
Abstract
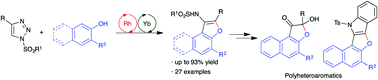
An efficient cascade Rh(II) and Yb(III) catalyzed transannulation of N-sulfonyl-1,2,3-triazoles with β-naphthols has been accomplished for the synthesis of substituted naphthofurans in good yields. The reaction involves the initial rhodium catalyzed insertion of azavinyl carbene into the O–H bond followed by ytterbium catalyzed annulative C–C bond formation and concomitant aerial oxidation. The developed reaction was successfully extended to phenols to afford substituted benzofurans. Furthermore, the synthetic utility of the method was demonstrated through the synthesis of important polyhetero aromatic compounds.


 Please wait while we load your content...
Please wait while we load your content...