Ligand-controlled iridium-catalyzed semihydrogenation of alkynes with ethanol: highly stereoselective synthesis of E- and Z-alkenes†
Abstract
A ligand-controlled iridium-catalyzed semihydrogenation of alkynes to E- and Z-alkenes with ethanol was developed. Effective selectivity control was achieved by ligand regulation. The use of 1,2-bis(diphenylphosphino)ethane (DPPE) and 1,5-cyclooctadiene (COD) was critical for the stereoselective semihydrogenation of alkynes. The general applicability of this procedure was highlighted by the synthesis of more than 40 alkenes, with good stereoselectivities. The value of our approach in practical applications was investigated by studying the effects of pinosylvin and 4,4′-dihydroxystilbene (DHS) on zebrafish as a vertebrate model.
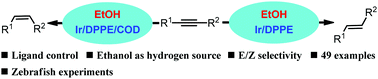


 Please wait while we load your content...
Please wait while we load your content...