Site-selective conversion of azido groups at carbonyl α-positions into oxime groups leading triazide to a triple click conjugation scaffold†
Abstract
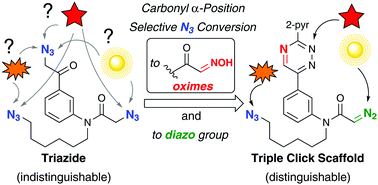
This paper reports the selective conversion of alkyl azido groups at the carbonyl α-position into oximes through β-elimination of dinitrogen, followed by transoximation. With this method and diazo conversion, a triazido molecule was transformed into a triple click conjugation scaffold allowing one-pot four-component coupling.


 Please wait while we load your content...
Please wait while we load your content...