A dimanganese(iii) porphyrin dication diradical and its transformation to a μ-hydroxo porphyrin–oxophlorin heterodimer†
Abstract
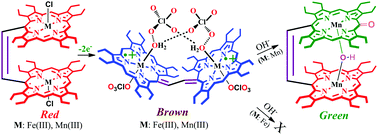
We investigate here the effect of electronic communication between two Mn(III) porphyrin π-cation radicals, connected covalently through an ethylene bridge, which exhibit significant electronic communication resulting in strong antiferromagnetic coupling predominantly between two porphyrin radical spins. This, however, is in sharp contrast to its diiron(III) analog where the porphyrin π-cation radical undergoes stronger antiferromagnetic coupling predominantly with the corresponding Fe(III) unpaired spin. Such a difference in the spin coupling model has also influenced their reactivity. While the dimanganese(III) dication diradical complex quickly transforms into a μ-hydroxo dimanganese(III) porphyrin–oxophlorin heterodimer upon addition of a base, its diiron(III) analog remains very stable.


 Please wait while we load your content...
Please wait while we load your content...