Unlocking high-potential non-persistent radical chemistry for semi-aqueous redox batteries†
Abstract
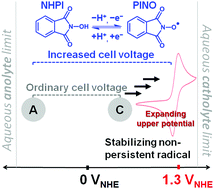
A non-persistent radical precursor, N-hydroxyphthalimide (NHPI), is reported as a low-cost, high-potential organic cathode in a binary electrolyte for a semi-aqueous redox battery. A highly reversible NHPI-phthalimide N-oxyl (PINO) radical redox couple at +1.30 VNHE is demonstrated, providing a 1.15 V rechargeable battery with an attractive >85% voltage efficiency when coupled with anthraquinone-2-sulfonic acid (AQS).


 Please wait while we load your content...
Please wait while we load your content...