Electrochemical CO2 reduction by a cobalt bipyricorrole complex: decrease of an overpotential value derived from monoanionic ligand character of the porphyrinoid species†
Abstract
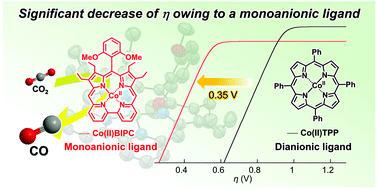
A newly synthesized Co(II) complex with a monoanionic bipyricorrole ligand is found to catalytically promote a selective CO2 electroreduction to CO with Faradaic efficiency of 75%. Catalytic Tafel plots show that the overpotential of Co(II) bipyricorrole is 0.35 V lower than that of a Co(II) complex with the dianionic tetraphenylporphyrin ligand.


 Please wait while we load your content...
Please wait while we load your content...
