Bioinspired radical cyclization of tryptamines: synthesis of peroxypyrroloindolenines as potential anti-cancer agents†
Abstract
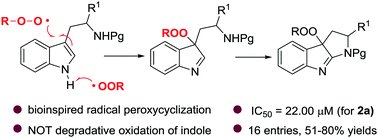
Inspired by the heme iron-catalyzed radical insertion of dioxygen to the tryptophan indole ring, herein we utilize alkylperoxy radical species as a coupling partner to trigger a peroxycyclization of readily accessible tryptophan derivatives and enable the first synthesis of peroxypyrroloindolenines. A preliminary biological evaluation revealed promising anti-cancer activities (IC50 = 22.00 μM for compound 2a), and revealed that both the indolenine core and the peroxy functionality are responsible for the antiproliferation effect against Hela cell lines.


 Please wait while we load your content...
Please wait while we load your content...