Facile reduction of carboxylic acids to primary alcohols under catalyst-free and solvent-free conditions†
Abstract
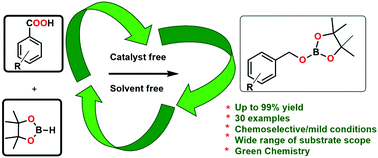
We report the development of a facile protocol for the deoxygenative hydroboration of aliphatic and aryl carboxylic acids to afford corresponding primary alcohols under solvent-free and catalyst-free conditions. The reaction proceeds under ambient temperature exhibits good tolerance towards various functional groups and generates quantitative yields. The plausible mechanism involves the formation of Lewis acid–base adducts as well as the liberation of hydrogen gas.
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm


 Please wait while we load your content...
Please wait while we load your content...