Dealkylative intercepted rearrangement reactions of sulfur ylides†
Abstract
Sulfur ylides are well-known to undergo sigmatropic rearrangement reaction. Herein, we describe a novel reactivity of sulfur ylides, which provides access to the product of a formal functional group metathesis upon dealkylative interception of the rearrangement process. Using a simple iron catalyst and in situ generated diazoalkanes this method provides access to α-mercaptoacetonitrile derivatives.
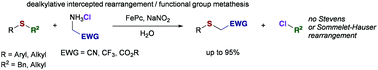


 Please wait while we load your content...
Please wait while we load your content...