Oxidative NHC catalysis: direct activation of β sp3 carbons of saturated acid chlorides†
Abstract
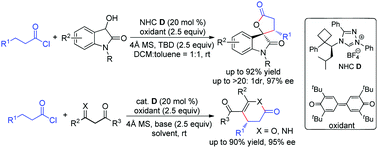
The first activation of saturated acid chlorides by oxidative N-heterocyclic carbene catalysis has been successfully utilized to synthesize enantio-enriched spirooxindole lactones and δ-lactones. The reaction involves the transformation of the β sp3 carbon of saturated acid chlorides into an electrophilic carbon as a key step. The product was obtained in excellent yield and stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...