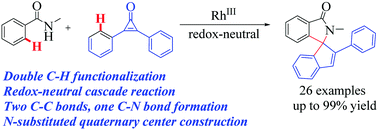
Rapid assembly of cyclopentene spiroisoindolinones via a rhodium-catalysed redox-neutral cascade reaction†
Abstract
An unprecedented strategy of a rhodium-catalysed redox-neutral cascade reaction starting from benzamides and cyclopropenones via addition of a C–H bond into a polar carbonyl group for the rapid assembly of cyclopentene spiroisoindolinones has been developed. Water was produced as the sole by-product. Metal migration and abstraction of a H-atom from the arylcyclopropenone's aromatic ring are the key steps in building the spirocycle. Two C–C bonds, one C–N bond and an N-substituted quaternary carbon center were formed sequentially in a one pot manner.


 Please wait while we load your content...
Please wait while we load your content...