Protein–protein inhibitor designed de novo to target the MEEVD region on the C-terminus of Hsp90 and block co-chaperone activity†
Abstract
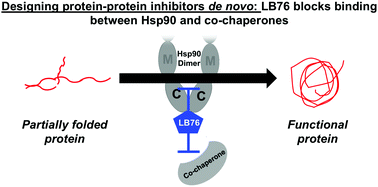
Protein–protein interactions control all cellular functions. Presented is the first de novo designed protein–protein inhibitor that targets the C-terminus of heat shock protein 90 (Hsp90) and blocks co-chaperones from binding. Compound LB76, which was created from an Hsp90 co-chaperone, selectively pulls down Hsp90 from cell lysates, binds to Hsp90's C-terminal domain, and blocks the interactions between Hsp90 and TPR-containing co-chaperones. Through these interactions, LB76 inhibits the protein-folding function of Hsp90. Blocking these protein–protein interactions between Hsp90 and C-terminal co-chaperones regulate the cell's entire protein-folding machinery.


 Please wait while we load your content...
Please wait while we load your content...