Solvent-free lithium and sodium containing electrolytes based on pseudo-delocalized anions†
Abstract
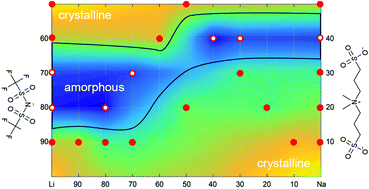
Mixing the standard battery salt LiTFSI with various Li-salts of novel pseudo-delocalized organic anions [N(CH3)2((CH2)nSO3)((CH2)mSO3)]− (MMnm11), results in super-cooled solvent-free liquid electrolytes with glass transition temperatures of ca. 50 °C. Synthesis routes and full chemical characterisation of the new pseudo-delocalized anions are presented, as well as phase and thermal stabilities. The ion conductivities and electrochemical stabilities are evaluated towards lithium and sodium battery application.


 Please wait while we load your content...
Please wait while we load your content...