Hydride transfer initiated ring expansion of pyrrolidines toward highly functionalized tetrahydro-1-benzazepines†
Abstract
A novel hydride transfer initiated ring expansion of 4-pyrrolidinyl isatins to synthesize tetrahydro-1-benzazepines has been developed. This methodology represents an atom- and step-economical protocol to assemble seven-membered polycyclic amines from pyrrolidine derivatives in one step.
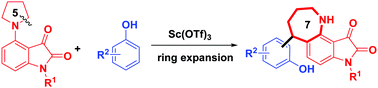


 Please wait while we load your content...
Please wait while we load your content...