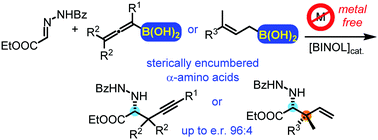
Catalytic asymmetric propargyl- and allylboration of hydrazonoesters: a metal-free approach to sterically encumbered chiral α-amino acid derivatives†
Abstract
A new asymmetric catalytic propargyl- and allylboration of hydrazonoesters is reported. The reactions utilize allenyl- and allylboronic acids in the presence of the inexpensive parent BINOL catalyst. The reactions can be performed under mild conditions (0 °C) without any metal catalyst or other additives affording sterically encumbered chiral α-amino acids. This is the first metal-free method for the asymmetric propargyl- and allylboration of hydrazonoesters.


 Please wait while we load your content...
Please wait while we load your content...