Construction of highly sterically hindered 1,1-disilylated terminal alkenes†
Abstract
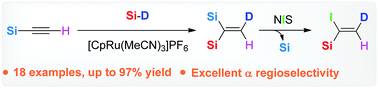
One direct and efficient procedure for the synthesis of 1,1-disilylated terminal alkenes is demonstrated in this paper. To overcome and rationally utilize the steric hindrance of silyl units, the cationic ruthenium catalyst [CpRu(MeCN)3]+ was found to be effective for Markovnikov hydrosilylation of 1-silyl terminal alkynes with high yields and excellent regioselectivity. Dissimilarities between alkyl and alkoxy silyl units lead to versatile product derivatizations toward a variety of useful building blocks.


 Please wait while we load your content...
Please wait while we load your content...