A single but hydrogen-bonded water molecule confined in an anisotropic subnanospace†
Abstract
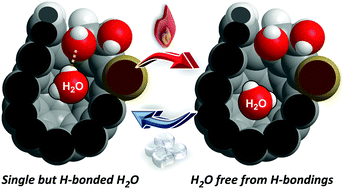
We have synthesized an open-cage C60 derivative having three hydroxy groups arranged in close proximity on the opening, which realized a single but hydrogen-bonded water molecule inside its cavity. The confined H2O molecule showed characteristic translational and rotational motions via the formation/cleavage of H-bonds. It is worth noting that the confined H2O molecule is less acidic and less basic than bulk water.


 Please wait while we load your content...
Please wait while we load your content...