Cyclopentannulation and cyclodehydrogenation of isomerically pure 5,11-dibromo-anthradithiophenes leading to contorted aromatics†
Abstract
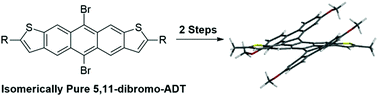
Isomerically pure 5,11-dibromo-2,8-dihexylanthra[2,3-b:76-b′]dithiophene, a brominated analog of anthracenedithiophene (ADT), was prepared and utilized for a palladium catalyzed cyclopentannulation reaction with 3,3′-dimethoxy-phenylacetylene to give cyclopentannulated ADT (CP-ADTs). A further Scholl cyclodehydrogenation reaction gave contorted aromatics with large splay angles, low optical gaps, and low LUMOs.


 Please wait while we load your content...
Please wait while we load your content...
