Neutral and anionic phosphate-diesters as molecular templates for the encapsulation of a water dimer†
Abstract
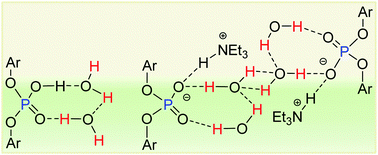
The study of water clusters in a confined state has received considerable attention. The simplest among such assemblies of water molecules is the water dimer. Stabilizing this unit at ambient conditions in an appropriate host-template, however, has remained a challenge. Herein, we report 2,6-(diphenylmethyl)-4-iso-propyl-phenyl substituted phosphate diesters (both in their neutral and anionic forms) as molecular templates for hosting the water dimer. The robustness of the water dimer within the neutral phosphate-diester ensemble is remarkable. Even when treated with triethylamine the water dimer is not disrupted, while exclusively the P(O)(OH) unit is deprotonated.


 Please wait while we load your content...
Please wait while we load your content...