Asymmetric synthesis via stereospecific C–N and C–O bond activation of alkyl amine and alcohol derivatives
Abstract
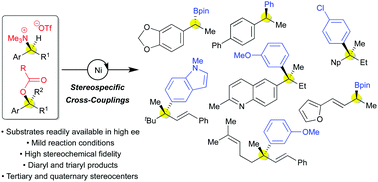
This perspective showcases our development of benzylic and allylic amine and alcohol derivatives as electrophiles for stereospecific, nickel-catalyzed cross-coupling reactions, as well as the prior art that inspired our efforts. The success of our effort has relied on the use of benzyl ammonium triflates as electrophiles for cross-couplings via C–N bond activation and benzylic and allylic carboxylates for cross-couplings via C–O bond activation. Our work, along with others’ exciting discoveries, has demonstrated the potential of stereospecific, nickel-catalyzed cross-couplings of alkyl electrophiles in asymmetric synthesis, and enables efficient generation of both tertiary and quaternary stereocenters.


 Please wait while we load your content...
Please wait while we load your content...