Iron-catalyzed synthesis of cyclopropanes by in situ generation and decomposition of electronically diversified diazo compounds†
Abstract
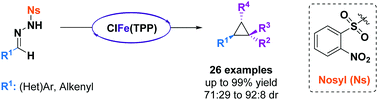
The modular synthesis of a variety of trans 1,2-disubstituted cyclopropanes in a safe and user-friendly one-pot iron-catalyzed cyclopropanation reaction is described. Easily synthesized N-nosylhydrazones are used as diazo precursors, allowing the in situ generation of electron-rich diazo compounds under mild reaction conditions and their direct participation in the cyclopropanation reaction.


 Please wait while we load your content...
Please wait while we load your content...