Lewis acid-catalyzed Friedel–Crafts reactions toward highly versatile, α-quaternary oxime ethers†
Abstract
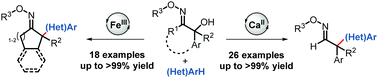
The synthesis of all-carbon-substituted, quaternary stereocenters through Lewis acid-catalyzed Friedel–Crafts alkylation of cyclic and acyclic 2-hydroxy oxime ethers proceeds under mild reaction conditions and with high yields. Moreover, the oxime ether moiety can be easily manipulated into various functional groups through subsequent modifications.


 Please wait while we load your content...
Please wait while we load your content...