Biomimetic synthesis of 2-substituted N-heterocycle alkaloids by one-pot hydrolysis, transamination and decarboxylative Mannich reaction†
Abstract
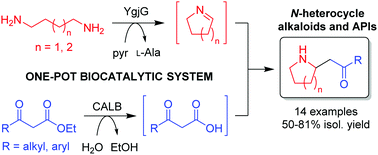
Heterocycles based on piperidine and pyrrolidine are key moieties in natural products and pharmaceutically active molecules. A novel multi-enzymatic approach based on the combination of a lipase with an α,ω-diamine transaminase is reported, opening up the synthesis, isolation and characterisation of a broad range of 2-substituted N-heterocycle alkaloids.


 Please wait while we load your content...
Please wait while we load your content...