Nickel-catalyzed, ligand-free, diastereoselective synthesis of 3-methyleneindan-1-ols†
Abstract
Nickel-catalyzed, highly diastereoselective annulations between activated allenes and 2-acetylarylboronic acid or 2-formylarylboronic acids are reported. No ligand for nickel is required, and the reactions proceed efficiently at room temperature to give a broad range of substituted 3-methyleneindan-1-ols. Preliminary results of an enantioselective variant are also described.
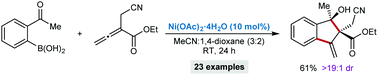


 Please wait while we load your content...
Please wait while we load your content...