Triazole-bearing calixpyrroles: strong halide binding affinities through multiple N–H and C–H hydrogen bonds†
Abstract
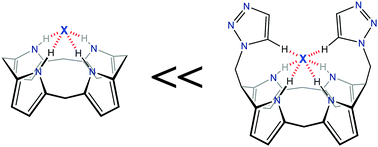
Triazole-bearing calixpyrroles (TCPs) were synthesized as artificial anion binding receptors. The additional C–H⋯X hydrogen bonding interaction induced strong binding affinity towards halide ions. Using this strong binding affinity, Cl− was successfully extracted from the aqueous to organic phase.


 Please wait while we load your content...
Please wait while we load your content...