Transition-metal-free multinitrogenation of amides by C–C bond cleavage: a new approach to tetrazoles†
Abstract
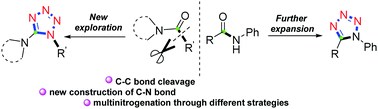
A metal-free brand-new one-pot multinitrogenation of amides for the chemo- and regioselective synthesis of 1,5-disubstituted tetrazoles has been developed. By means of electrophilic amide activation, and further C–C bond cleavage and rearrangement, a diverse set of functionalized 1,5-DST derivatives were selectively constructed under mild conditions. As showcased in the mechanisms, the chemoselectivity is easily switched by the selection of the starting materials in the reaction.


 Please wait while we load your content...
Please wait while we load your content...