Metal-free direct C-arylation of 1,3-dicarbonyl compounds and ethyl cyanoacetate: a platform to access diverse arrays of meta-functionalized phenols†
Abstract
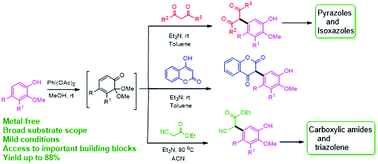
A base mediated, highly convenient strategy for the direct C-arylation of 1,3-dicarbonyls and cyanoacetate with various phenol derivatives as aryl partners is presented. The present work excels in forming a C–C bond at the meta-position of the phenols, which is traditionally challenging to functionalize. This protocol further leads the way to have scalable, straightforward access to phenol assimilated heterocycles which have powerful applications both in synthetic chemistry and medicinal research.


 Please wait while we load your content...
Please wait while we load your content...