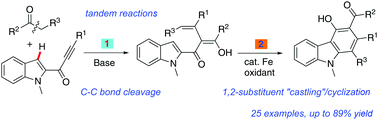
Merging base-promoted C–C bond cleavage and iron-catalyzed skeletal rearrangement involving C–C/C–H bond activation: synthesis of highly functionalized carbazoles†
Abstract
An efficient and atom-economical methodology for the synthesis of multi-substituted carbazoles starting from α-aryl ketones and ynones under mild reaction conditions has been developed. This process goes through Cs2CO3 promoted C–C σ-bond activation of α-aryl ketones followed by highly selective C–H bond activations and C–C bond fragmentations in a one-pot operation.


 Please wait while we load your content...
Please wait while we load your content...