Roles of soluble species in the alkaline oxygen evolution reaction on a nickel anode†
Abstract
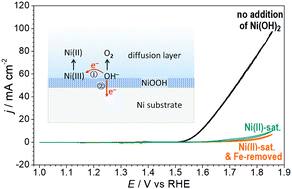
The roles of soluble Fe and Ni species in the alkaline oxygen evolution reaction (OER) at a Ni anode are reported. The Fe impurities in the electrolyte turn out to be insufficient to directly improve the OER activity. The Ni(OH)2/NiOOH film undergoes chemical dissolution to give a stable Ni(II) species that plays a hindering role in the OER.


 Please wait while we load your content...
Please wait while we load your content...