Recent advances in the sulfonylation of alkenes with the insertion of sulfur dioxide via radical reactions
Abstract
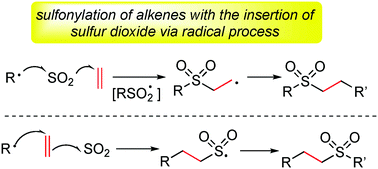
Recent advances in the sulfonylation of alkenes via the insertion of sulfur dioxide are summarized. The sulfur dioxide surrogate of DABCO·(SO2)2 or inorganic sulfites is used in the transformation through a radical process. Two strategies for the sulfonylation of alkenes with the insertion of sulfur dioxide have been developed. In most cases, the vicinal difunctionalization of alkenes is initiated by the arylsulfonyl radical generated in situ. Although the sulfonylation of alkenes with the insertion of sulfur dioxide is efficient, the asymmetric versions are still challenging and less to be explored. It is expected that the asymmetric vicinal difunctionalization of alkenes with the insertion of sulfur dioxide will be developed rapidly in the near future.


 Please wait while we load your content...
Please wait while we load your content...