Quantification of the liquid window of deep eutectic solvents†
Abstract
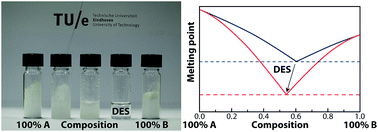
Deep eutectic solvents (DESs) have been considered as a new class of green solvents with tunable physical properties based on the selective combination of their individual components. As the liquid window of a DES identifies the range of feasible applications, it is essential to determine, quantify, and predict their phase behavior. Phase diagrams were measured for systems consisting of tetrapentylammonium bromide and erythritol or succinic acid. Regular solution theory is applied to quantitatively describe the liquid window of DESs. The succinic acid mixture shows a larger deviation from ideal behavior, caused by the stronger hydrogen bond forming acid groups. The interaction parameter between the two DES components in regular solution theory could be determined directly from the eutectic temperature of the mixture and this enables quantification of the degree of non-ideality of DESs.


 Please wait while we load your content...
Please wait while we load your content...