A comparative DFT study of TBD-catalyzed reactions of amines with CO2 and hydrosilane: the effect of solvent polarity on the mechanistic preference and the origins of chemoselectivities†
Abstract
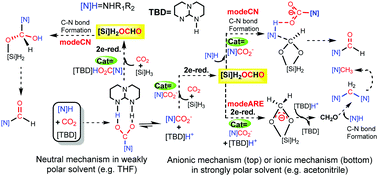
A DFT comparative mechanistic study unveils that the TBD-catalyzed reactions of amines with CO2 and hydrosilanes may either undergo a neutral mechanism or a mechanism involving free ions, depending on the polarity of the solvent. The nucleophilicity of the amines is an important factor to determine the chemoselectivities of the reactions to give formamide or aminal/N-methylated amine.


 Please wait while we load your content...
Please wait while we load your content...