Catalyst-free geminal aminofluorination of ortho-sulfonamide-tethered alkylidenecyclopropanes via a Wagner–Meerwein rearrangement†
Abstract
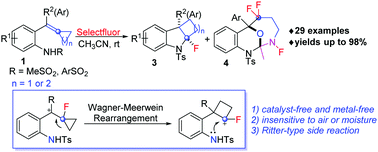
A catalyst-free intramolecular geminal aminofluorination of ortho-sulfonamide-tethered alkylidenecyclopropanes has been developed. The reaction proceeded through two SET processes with Selectfluor to give a fluorinated cyclopropylcarbinyl cation and a further Wagner-Meerwein rearrangement to generate a cyclobutyl carbocation, which undergoes intramolecular nucleophilic capture by amide to forge fluorinated cyclobuta[b]indoline derivatives. A polycyclic multi-fluorinated byproduct was also formed through a Ritter-type reaction in some cases.


 Please wait while we load your content...
Please wait while we load your content...