Enantioselective synthesis of axially chiral vinyl arenes through palladium-catalyzed C–H olefination†
Abstract
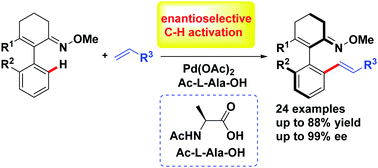
A palladium-catalyzed ketoxime-chelation-assisted enantioselective C–H olefination of 2-arylcyclohex-2-enone oxime ether with a wide range of olefins as coupling partners was developed. Employing ketoxime ether as an efficient directing group, a variety of axially chiral vinyl arenes was synthesized by Pd(II)-catalyzed C–H functionalization with excellent enantioselectivities (96 → 99% ee) under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...