Chiral phosphoric acid catalyzed enantioselective N-alkylation of indoles with in situ generated cyclic N-acyl ketimines†
Abstract
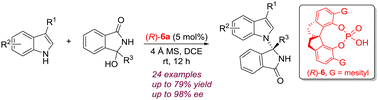
A chiral SPINOL derived phosphoric acid-catalyzed asymmetric N-alkylation reaction of indoles with cyclic α-diaryl-substituted N-acyl imines, which are generated in situ from 3-aryl 3-hydroxyisoindolinones, has been demonstrated. The transformation proceeds smoothly with a broad range of indoles and isoindolinone alcohols. A variety of indole derived N-alkylated tetrasubstituted chiral aminals were afforded in moderate to good yields (50–79%) with excellent enantioselectivities (up to 98% ee).


 Please wait while we load your content...
Please wait while we load your content...