Engineering a cleavable disulfide bond into a natural product siderophore using precursor-directed biosynthesis†
Abstract
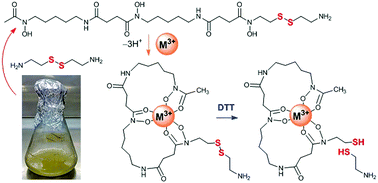
An analogue of the bacterial siderophore desferrioxamine B (DFOB) containing a disulfide motif in the backbone was produced from Streptomyces pilosus cultures supplemented with cystamine. Cystamine competed against native 1,5-diaminopentane during assembly. DFOB-(SS)1[001] and its complexes with Fe(III) or Ga(III) were cleaved upon incubation with dithiothreitol. Compounds such as DFOB-(SS)1[001] and its thiol-containing cleavage products could expand antibiotic strategies and Au–S-based nanotechnologies.


 Please wait while we load your content...
Please wait while we load your content...
