Squaryl group modified phosphoglycolipid analogs as potential modulators of GPR55†
Abstract
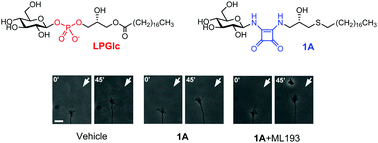
Lysophosphatidyl glucoside (LPGlc) is a structurally unique glycolipid that acts as a guidance cue for extending axons during central nervous system development by activating the class A G protein coupled receptor (GPR) 55 of spinal cord sensory axons. GPR55 not only plays an important role during development, but is also implicated in many disease states, rendering molecules that target GPR55 of widespread interest. In this study, we developed synthetic access to a novel class of LPGlc analogues featuring a squaryl diamide group as surrogate for the phosphodiester. We report the facile synthesis of a series of LPGlc analogues, their GPR dependent biological activity and a systematic analysis of the structure–activity relationship in regards to GPR55 modulation. The lead compound featuring identical configuration at all stereocenters compared to natural LPGlc exhibits an activity to repel axons of dorsal root ganglion (DGR) nociceptive neurons.


 Please wait while we load your content...
Please wait while we load your content...