Binding and backbone dynamics of protein under topological constraint: calmodulin as a model system†
Abstract
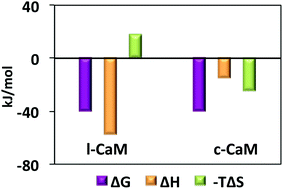
Herein we present the effect of artificially imposed topological constraint on calmodulin (CaM) backbone dynamics and its molecular recognition behavior. While backbone dynamics of CaM remain largely unperturbed, the thermodynamic profile of CaM binding to the smooth-muscle myosin light-chain kinase (smMLCK) peptide is modulated significantly.


 Please wait while we load your content...
Please wait while we load your content...