Steric vs. electronic stereocontrol in syndio- or iso-selective ROP of functional chiral β-lactones mediated by achiral yttrium-bisphenolate complexes
Abstract
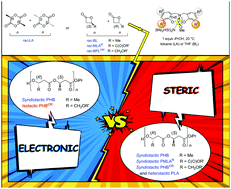
Origins of stereoselectivity in ROP of racemic chiral cyclic esters promoted by achiral yttrium complexes, resulting in the formation of highly heterotactic polylactide, and highly syndiotactic or, more uniquely, highly isotactic poly(3-hydroxybutyrate)s, are discussed. A close interplay between the nature of the cyclic ester, most particularly of the exocyclic functional chain installed on the chiral center of β-lactones, and the ortho-substituents installed on the phenolate rings of the ligand, results in various determining secondary interactions of steric and also electronic nature.
- This article is part of the themed collection: New molecules and materials from the f-block


 Please wait while we load your content...
Please wait while we load your content...