Asymmetric total synthesis of (+)-ovafolinins A and B†
Abstract
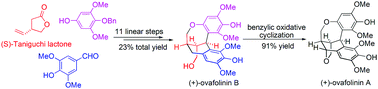
(+)-Ovafolinins A and B are two homologous lignans containing unique polycyclic skeletons. Benefiting from a highly diastereoselective alkylation of (S)-Taniguchi lactone, a double Friedel–Crafts reaction, a global debenzylation and a Cu(OAc)2-enabled benzylic oxidative cyclization, we present herein an efficient synthetic approach to (+)-ovafolinins A and B.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...