Enantioselective total synthesis of sagittacin E and related natural products†
Abstract
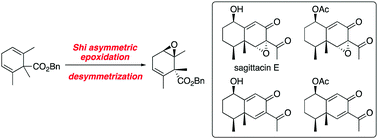
The first enantioselective total synthesis of eremophilane-type sesquiterpenoids, sagittacin E and related natural products, was achieved. This synthesis features an asymmetric desymmetrization by Shi asymmetric epoxidation, intramolecular aldol-type cyclization, allylic oxidation of a 1,4-diene compound, and stereoselective epoxidation.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...